
Enhancing Efficacy and Safety of 4- 1BB Agonism with Tumor-Targeted Bispecifics Ingmar Bruns, M.D., PH.D. Senior Vice President, Head of Clinical Development Pieris Pharmaceuticals, Inc. #IO360nyc

Forward Looking Statements This presentation contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, references to novel technologies and methods and our business and product development plans, including the advancement of our proprietary and co-development programs into and through the clinic. Actual results could differ from those projected in any forward-looking statements due to numerous factors. Such factors include, among others, our ability to raise the additional funding we will need to continue to pursue our business and product development plans; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; our ability to develop, complete clinical trials for, obtain approvals for and commercialize any of our product candidates, including our ability to recruit and enroll patients in our studies; our ability to address the requests of the FDA; competition in the industry in which we operate and market conditions. These forward-looking statements are made as of the date of this press release, and we assume no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Investors should consult all of the information set forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents we file with the SEC available at�www.sec.gov, including without limitation the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2018 and the Company's Quarterly Reports on Form 10-Q. #IO360nyc
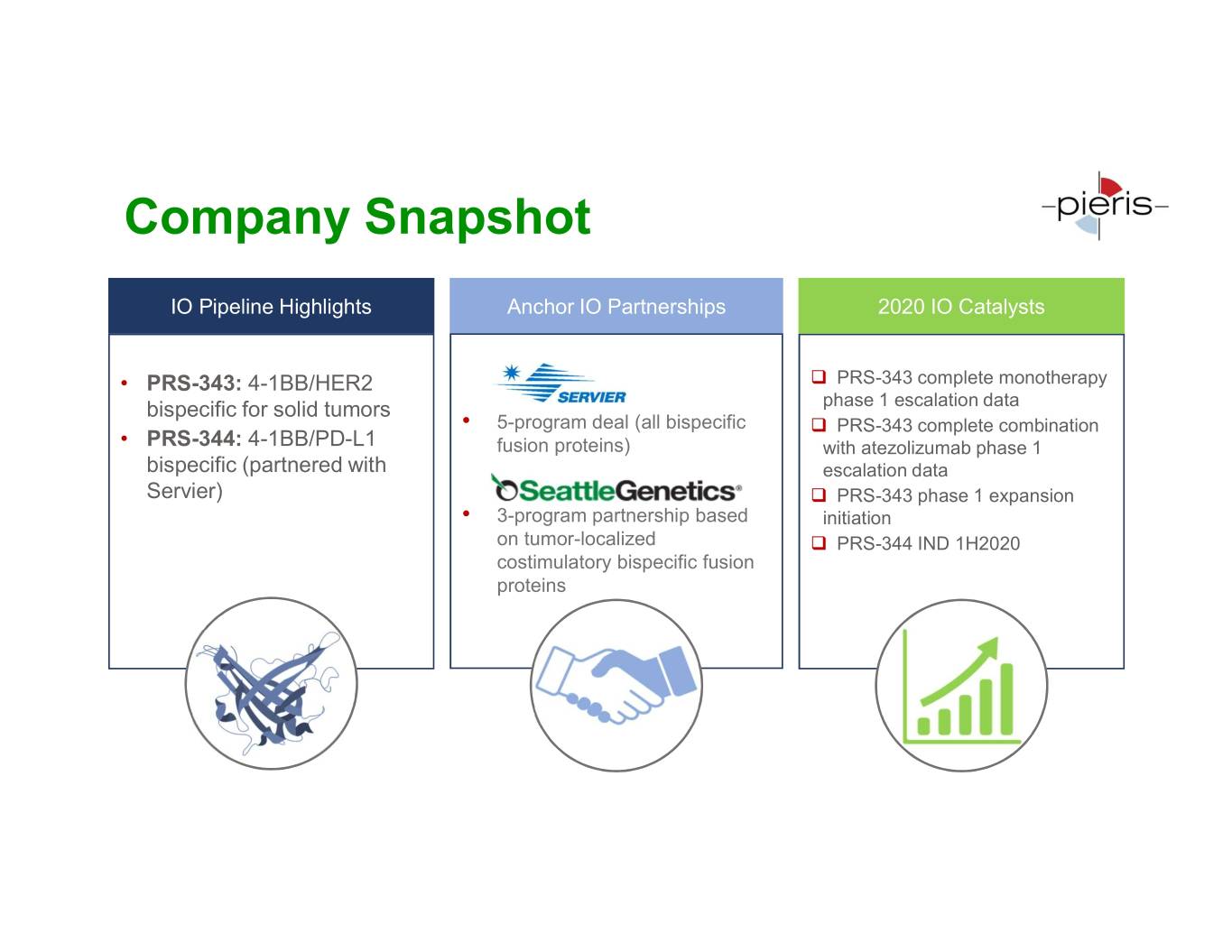
Company Snapshot IO Pipeline Highlights Anchor IO Partnerships 2020 IO Catalysts • PRS-343: 4-1BB/HER2 PRS-343 complete monotherapy bispecific for solid tumors phase 1 escalation data • 5-program deal (all bispecific PRS-343 complete combination • PRS-344: 4-1BB/PD-L1 fusion proteins) with atezolizumab phase 1 bispecific (partnered with escalation data Servier) PRS-343 phase 1 expansion • 3-program partnership based initiation on tumor-localized PRS-344 IND 1H2020 costimulatory bispecific fusion proteins
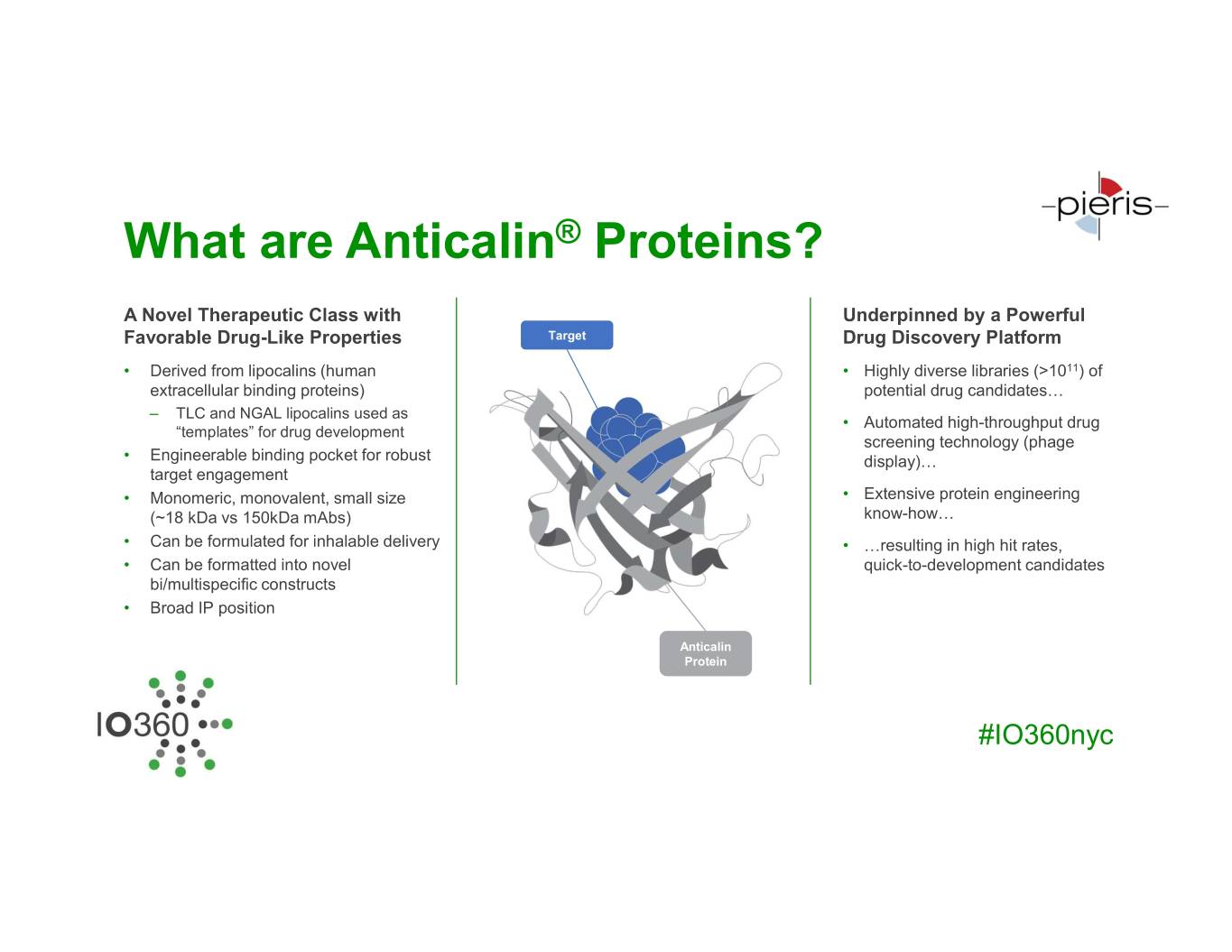
What are Anticalin® Proteins? A Novel Therapeutic Class with Underpinned by a Powerful Favorable Drug-Like Properties Target Drug Discovery Platform • Derived from lipocalins (human • Highly diverse libraries (>1011) of extracellular binding proteins) potential drug candidates… – TLC and NGAL lipocalins used as • Automated high-throughput drug “templates” for drug development screening technology (phage • Engineerable binding pocket for robust display)… target engagement • Monomeric, monovalent, small size • Extensive protein engineering (~18 kDa vs 150kDa mAbs) know-how… • Can be formulated for inhalable delivery • …resulting in high hit rates, • Can be formatted into novel quick-to-development candidates bi/multispecific constructs • Broad IP position Anticalin Protein #IO360nyc
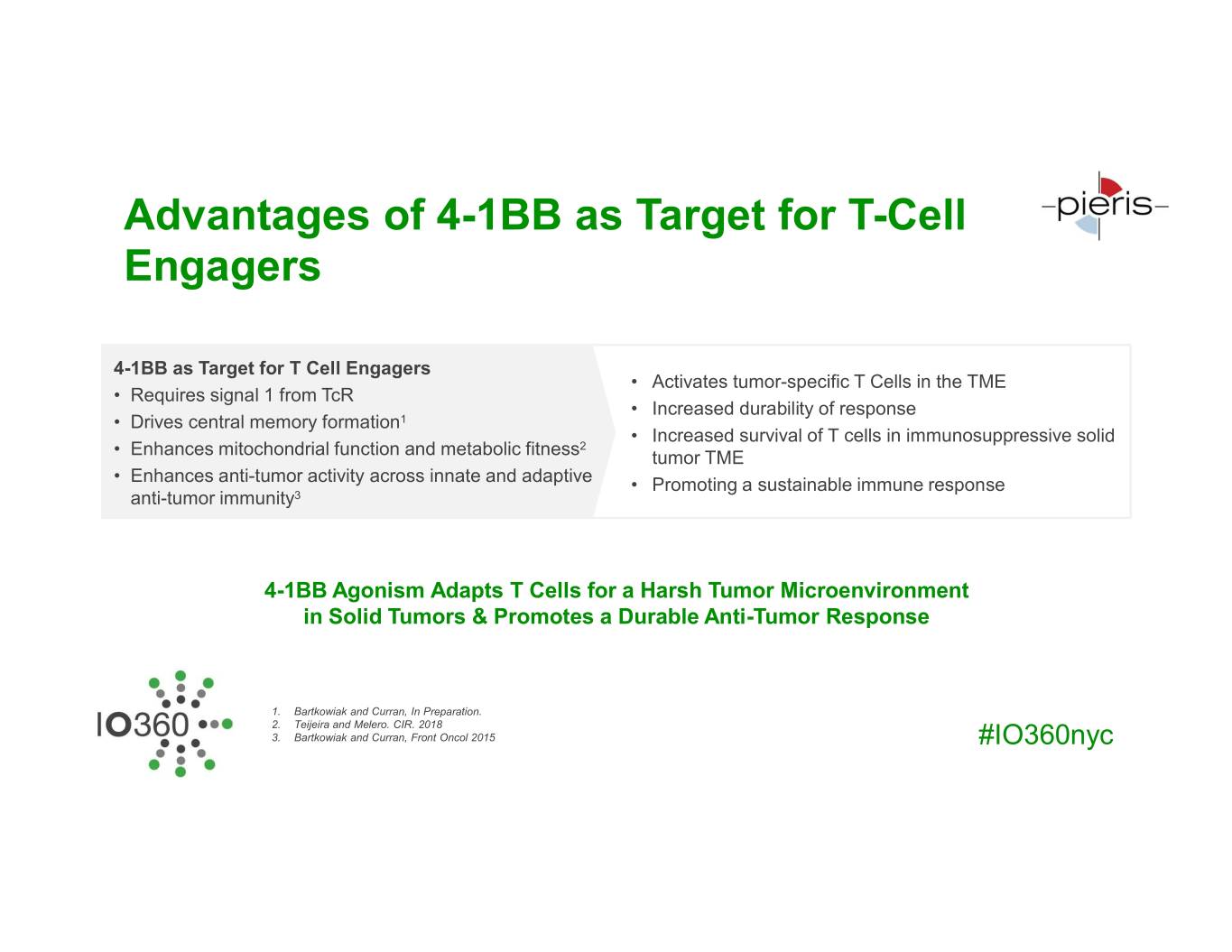
Advantages of 4-1BB as Target for T-Cell Engagers 4-1BB as Target for T Cell Engagers • Activates tumor-specific T Cells in the TME • Requires signal 1 from TcR • Increased durability of response • Drives central memory formation1 • Increased survival of T cells in immunosuppressive solid 2 • Enhances mitochondrial function and metabolic fitness tumor TME • Enhances anti-tumor activity across innate and adaptive • Promoting a sustainable immune response anti-tumor immunity3 4-1BB Agonism Adapts T Cells for a Harsh Tumor Microenvironment in Solid Tumors & Promotes a Durable Anti-Tumor Response 1. Bartkowiak and Curran, In Preparation. 2. Teijeira and Melero. CIR. 2018 3. Bartkowiak and Curran, Front Oncol 2015 #IO360nyc
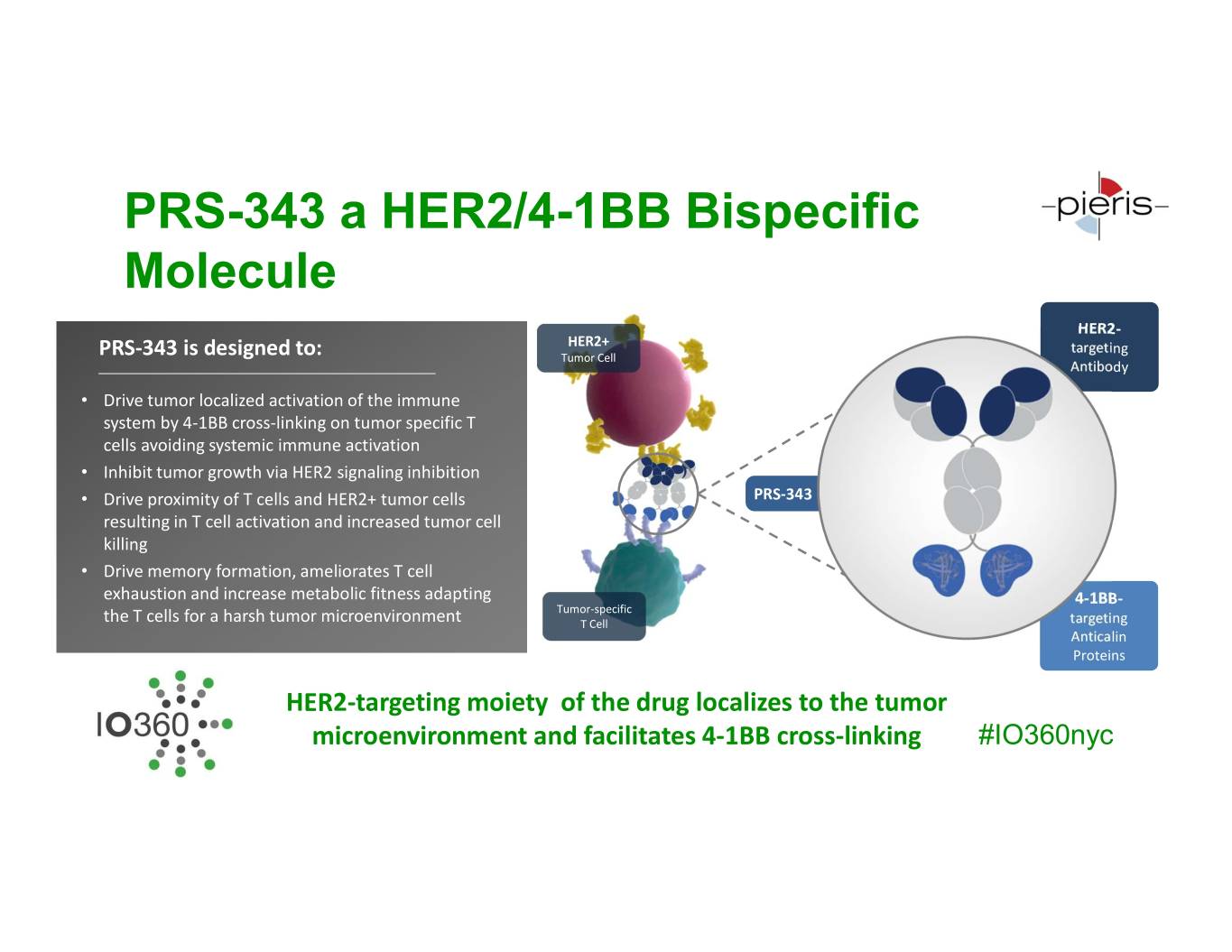
PRS-343 a HER2/4-1BB Bispecific Molecule HER2- HER2+ targeting PRS-343 is designed to: Tumor Cell Antibody • Drive tumor localized activation of the immune system by 4-1BB cross-linking on tumor specific T cells avoiding systemic immune activation • Inhibit tumor growth via HER2 signaling inhibition • Drive proximity of T cells and HER2+ tumor cells PRS-343 resulting in T cell activation and increased tumor cell killing • Drive memory formation, ameliorates T cell exhaustion and increase metabolic fitness adapting 4-1BB- Tumor-specific the T cells for a harsh tumor microenvironment T Cell targeting Anticalin Proteins HER2-targeting moiety of the drug localizes to the tumor microenvironment and facilitates 4-1BB cross-linking #IO360nyc
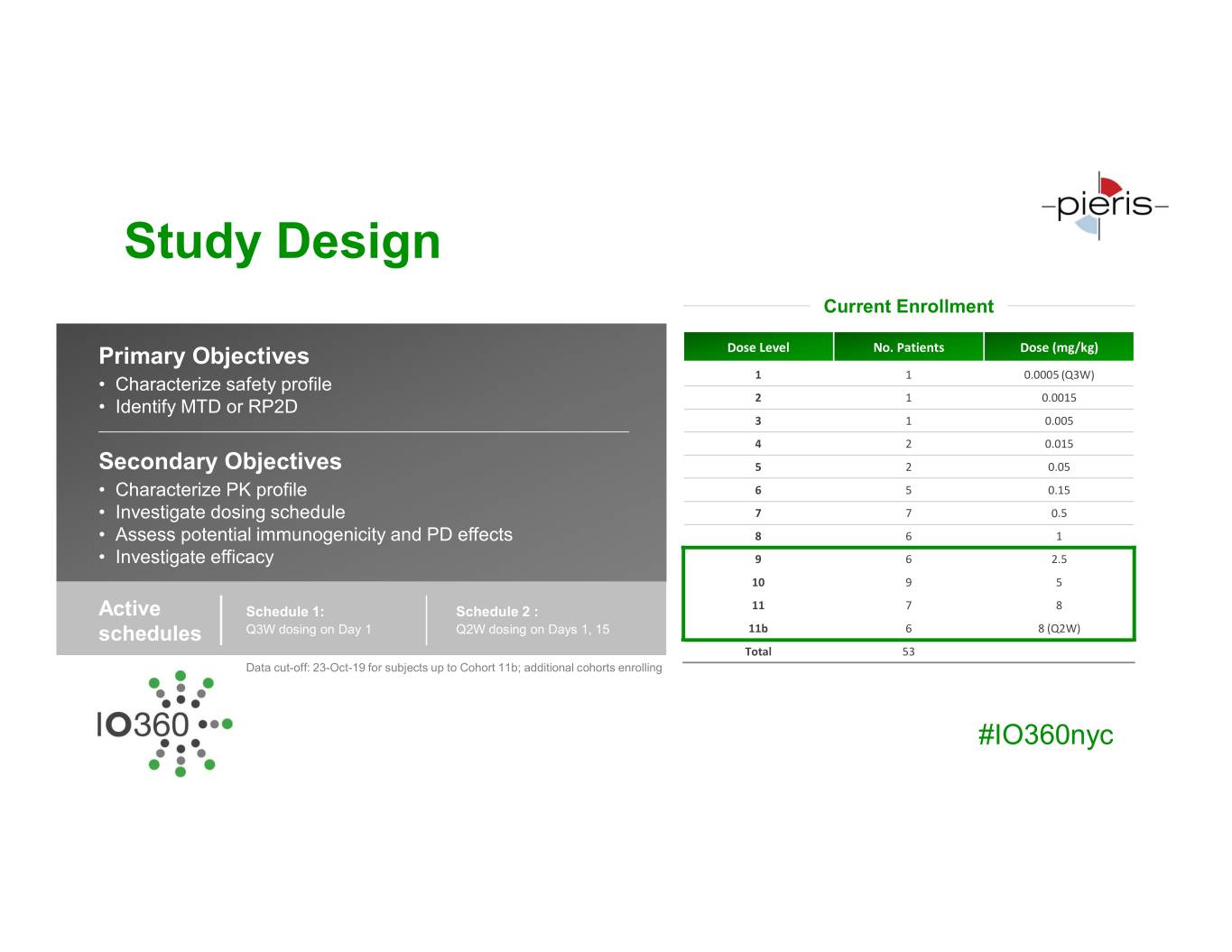
Study Design Current Enrollment Primary Objectives Dose Level No. Patients Dose (mg/kg) • Characterize safety profile 1 1 0.0005 (Q3W) • Identify MTD or RP2D 2 1 0.0015 3 1 0.005 4 2 0.015 Secondary Objectives 5 2 0.05 • Characterize PK profile 6 5 0.15 • Investigate dosing schedule 7 7 0.5 • Assess potential immunogenicity and PD effects 8 6 1 • Investigate efficacy 9 6 2.5 10 9 5 Active Schedule 1: Schedule 2 : 11 7 8 schedules Q3W dosing on Day 1 Q2W dosing on Days 1, 15 11b 6 8 (Q2W) Total 53 Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling #IO360nyc
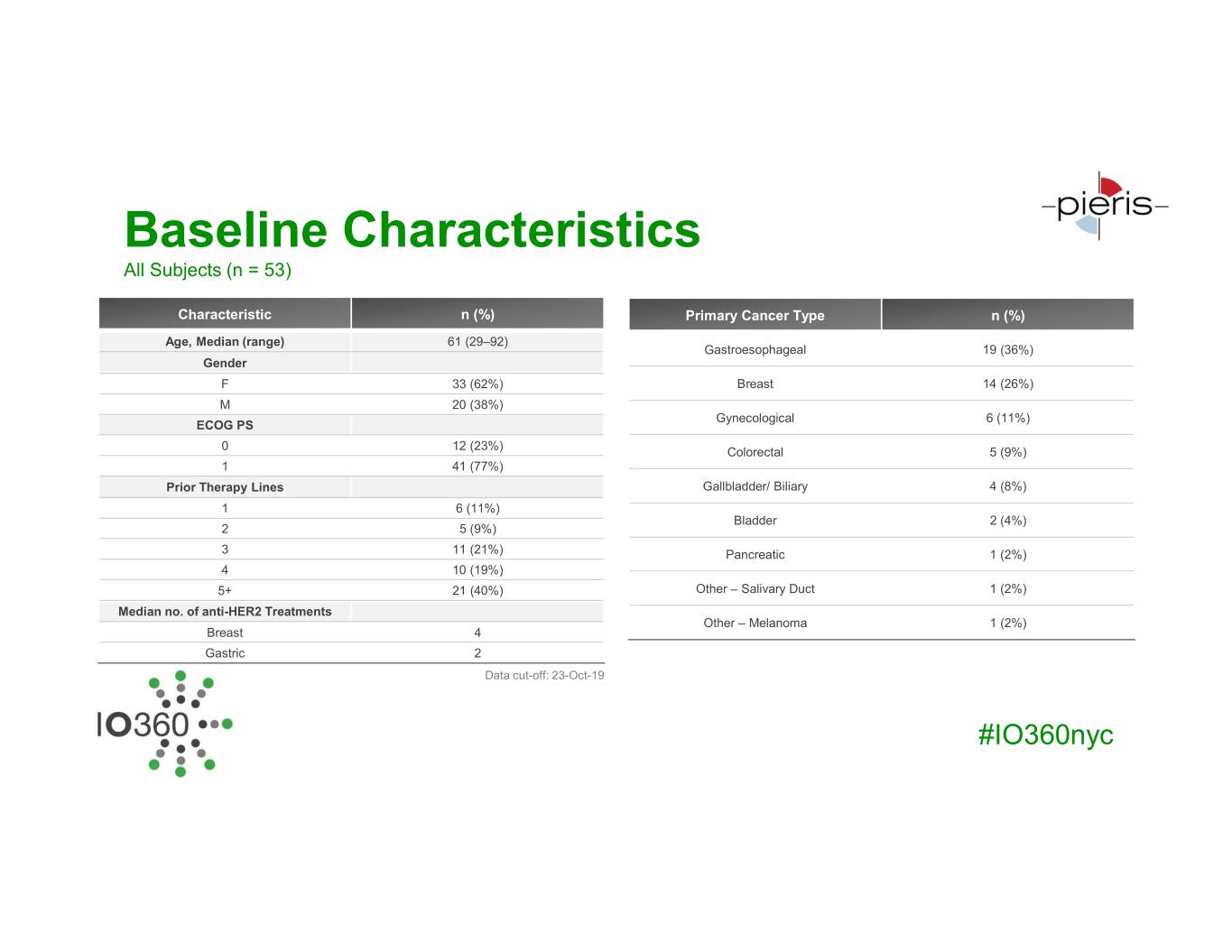
Baseline Characteristics All Subjects (n = 53) Characteristic n (%) Primary Cancer Type n (%) Age, Median (range) 61 (29–92) Gastroesophageal 19 (36%) Gender F 33 (62%) Breast 14 (26%) M 20 (38%) Gynecological 6 (11%) ECOG PS 0 12 (23%) Colorectal 5 (9%) 1 41 (77%) Prior Therapy Lines Gallbladder/ Biliary 4 (8%) 1 6 (11%) Bladder 2 (4%) 2 5 (9%) 3 11 (21%) Pancreatic 1 (2%) 4 10 (19%) 5+ 21 (40%) Other – Salivary Duct 1 (2%) Median no. of anti-HER2 Treatments Other – Melanoma 1 (2%) Breast 4 Gastric 2 Data cut-off: 23-Oct-19 #IO360nyc
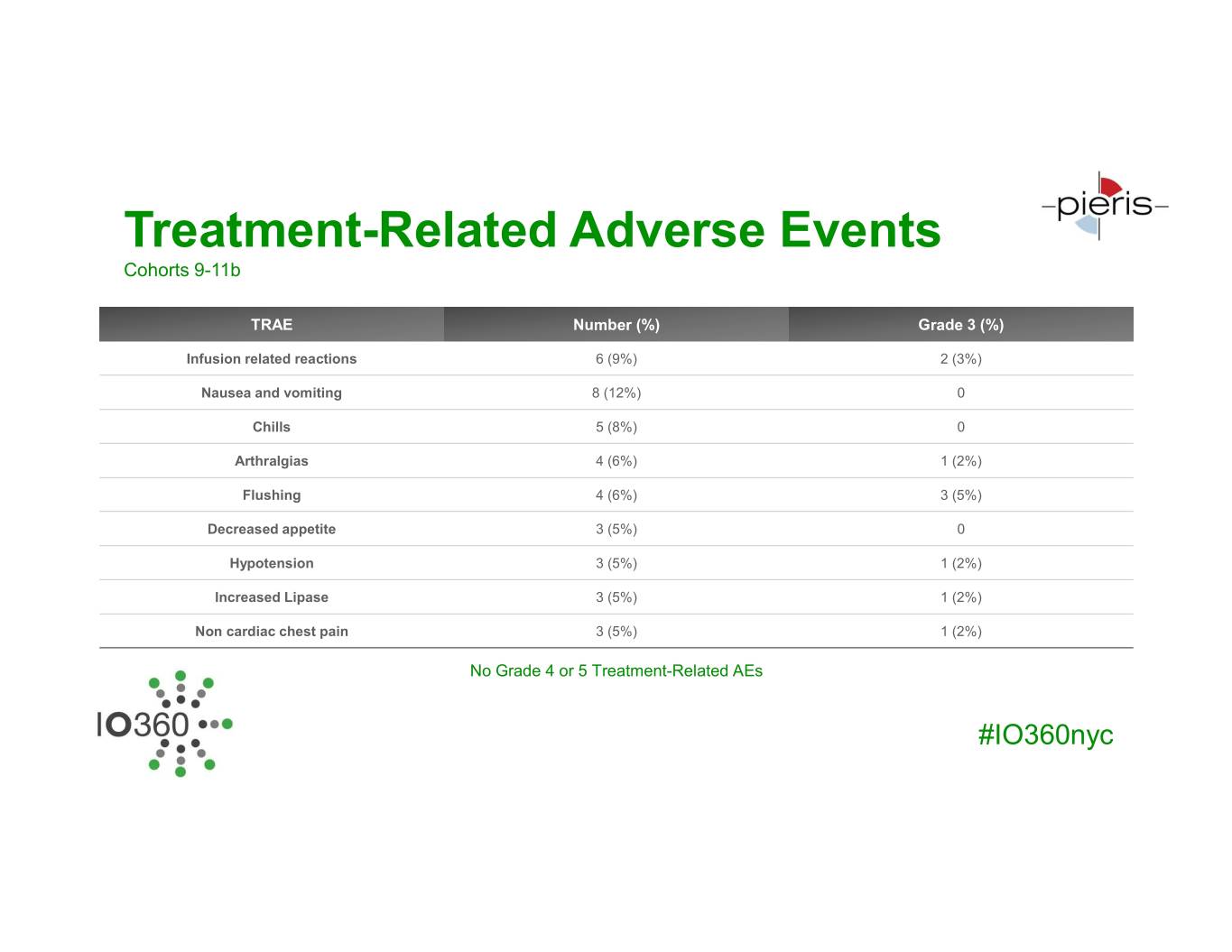
Treatment-Related Adverse Events Cohorts 9-11b TRAE Number (%) Grade 3 (%) Infusion related reactions 6 (9%) 2 (3%) Nausea and vomiting 8 (12%) 0 Chills 5 (8%) 0 Arthralgias 4 (6%) 1 (2%) Flushing 4 (6%) 3 (5%) Decreased appetite 3 (5%) 0 Hypotension 3 (5%) 1 (2%) Increased Lipase 3 (5%) 1 (2%) Non cardiac chest pain 3 (5%) 1 (2%) No Grade 4 or 5 Treatment-Related AEs #IO360nyc
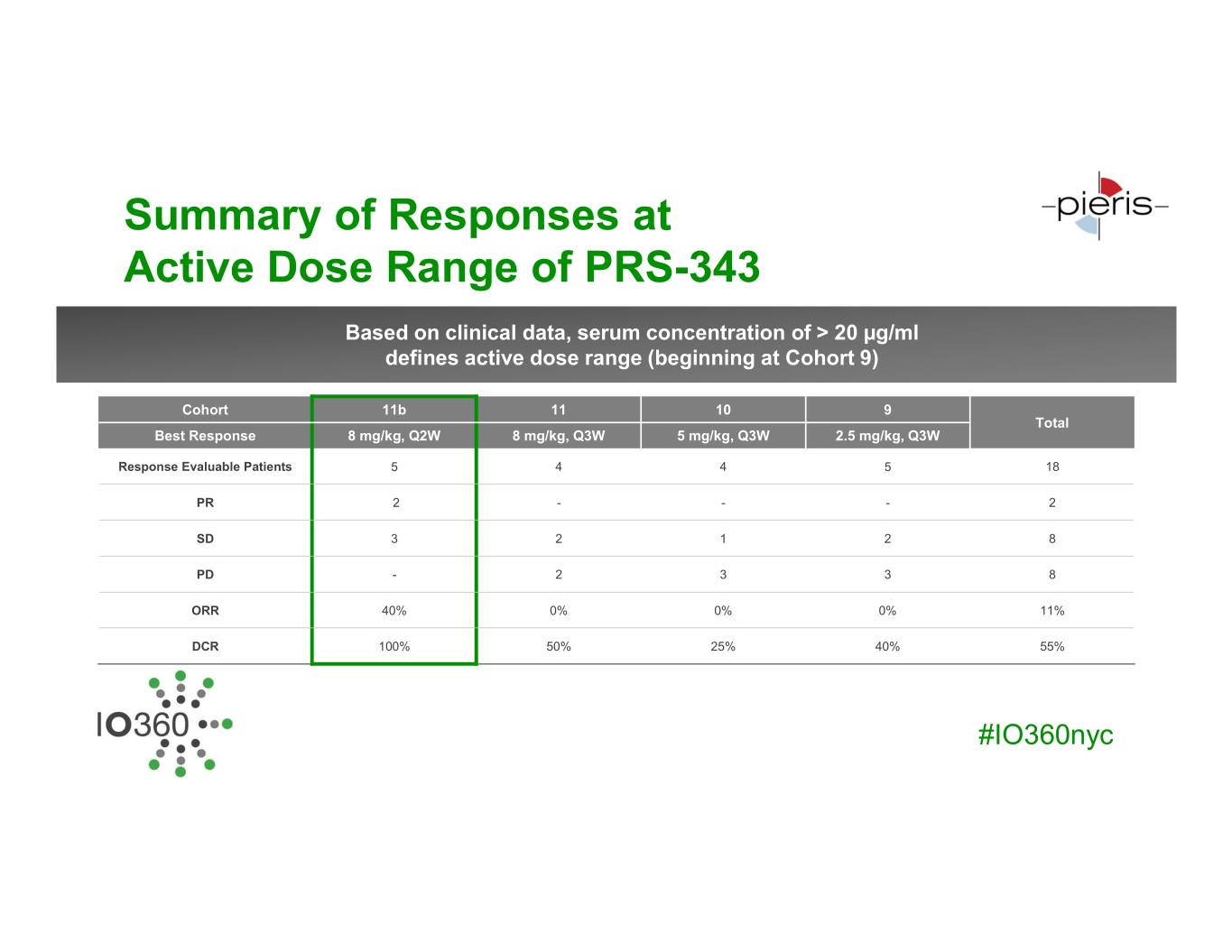
Summary of Responses at Active Dose Range of PRS-343 Based on clinical data, serum concentration of > 20 µg/ml defines active dose range (beginning at Cohort 9) Cohort 11b 11 10 9 Total Best Response 8 mg/kg, Q2W 8 mg/kg, Q3W 5 mg/kg, Q3W 2.5 mg/kg, Q3W Response Evaluable Patients 5 4 4 5 18 PR 2 - - - 2 SD 3 2 1 2 8 PD - 2 3 3 8 ORR 40% 0% 0% 0% 11% DCR 100% 50% 25% 40% 55% #IO360nyc
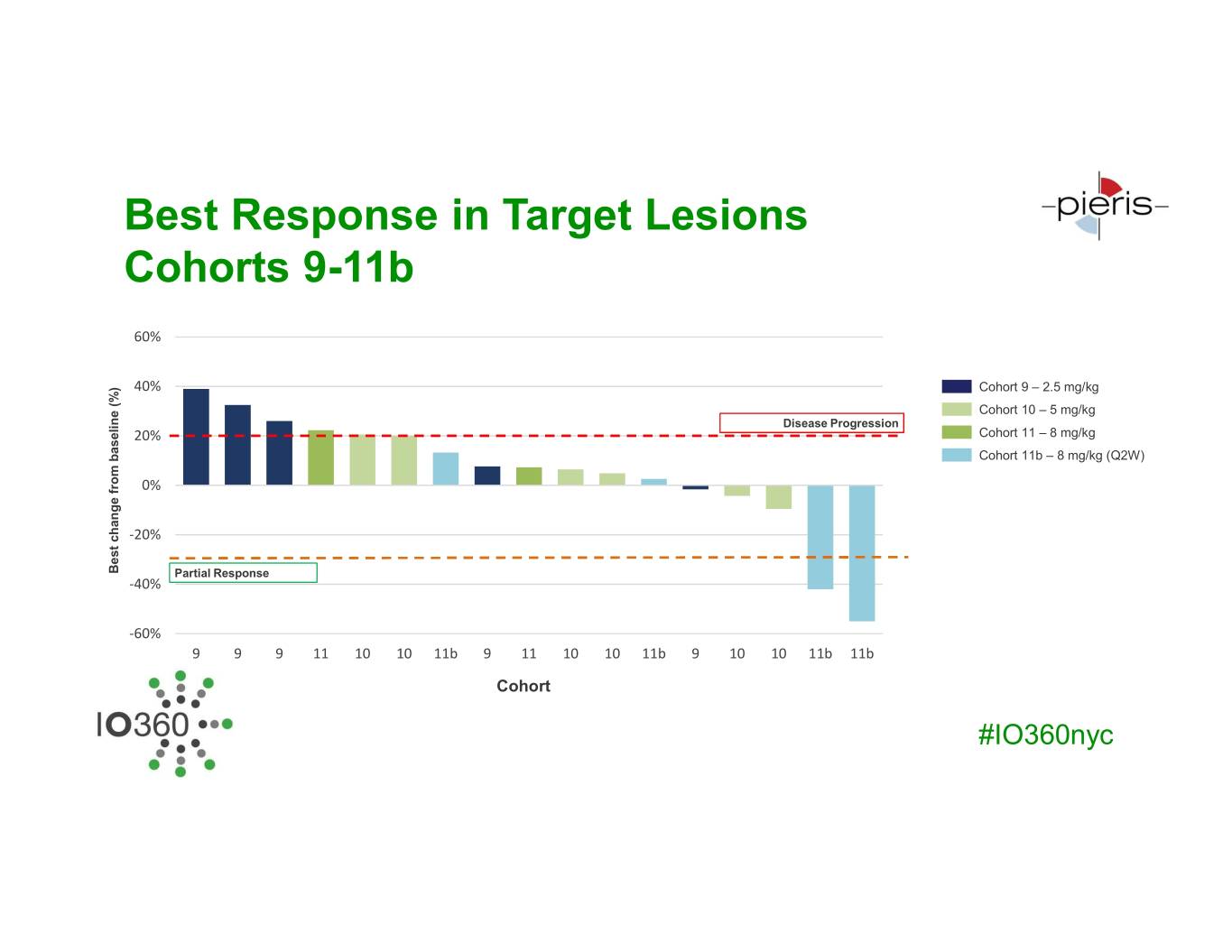
Best Response in Target Lesions Cohorts 9-11b 60% 40% Cohort 9 – 2.5 mg/kg Cohort 10 – 5 mg/kg Disease Progression 20% Cohort 11 – 8 mg/kg Cohort 11b – 8 mg/kg (Q2W) 0% -20% Best changeBest baselinefrom (%) Partial Response -40% -60% 9 9 9 11 10 10 11b 9 11 10 10 11b 9 10 10 11b 11b Cohort #IO360nyc
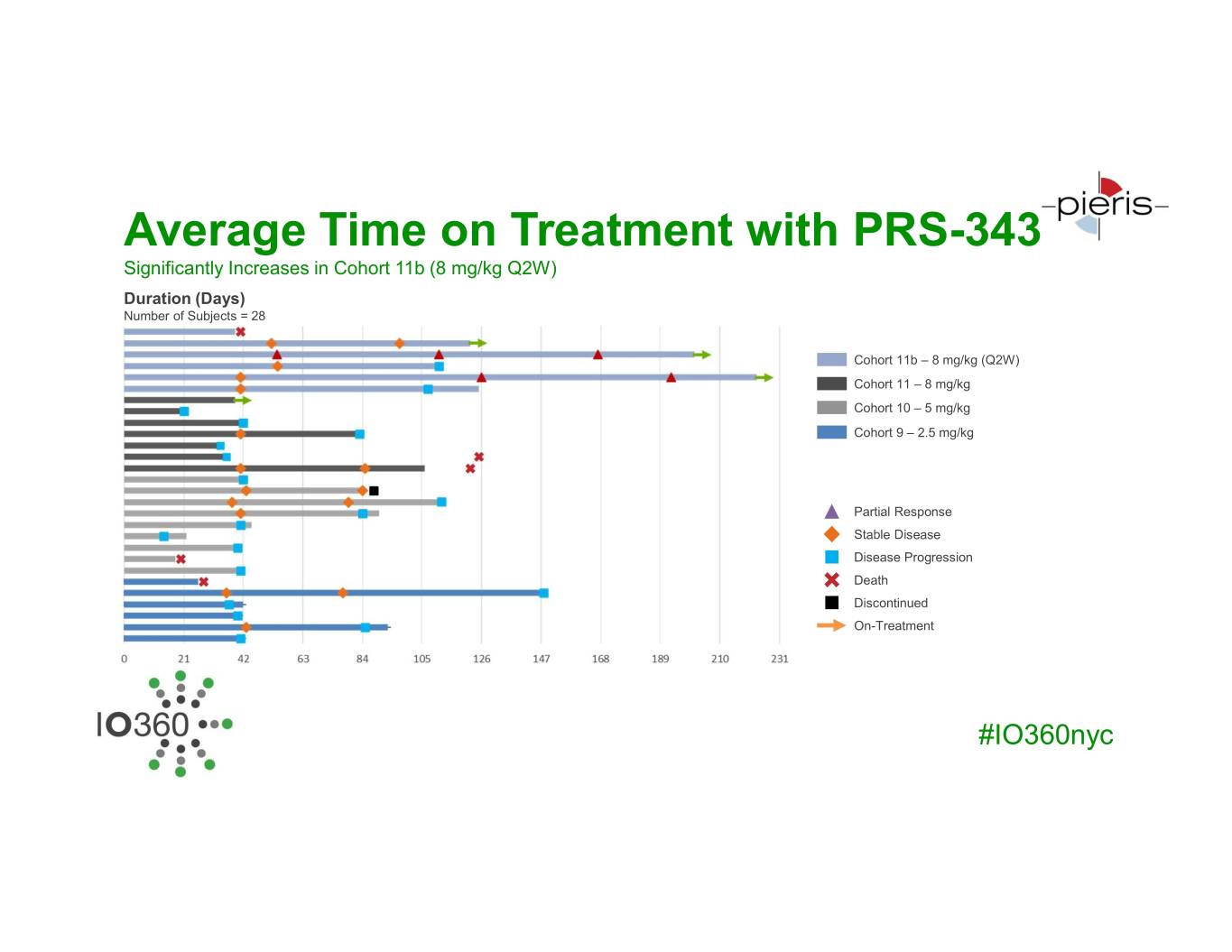
Average Time on Treatment with PRS-343 Significantly Increases in Cohort 11b (8 mg/kg Q2W) Duration (Days) Number of Subjects = 28 Cohort 11b – 8 mg/kg (Q2W) Cohort 11 – 8 mg/kg Cohort 10 – 5 mg/kg Cohort 9 – 2.5 mg/kg Partial Response Stable Disease Disease Progression Death Discontinued On-Treatment #IO360nyc
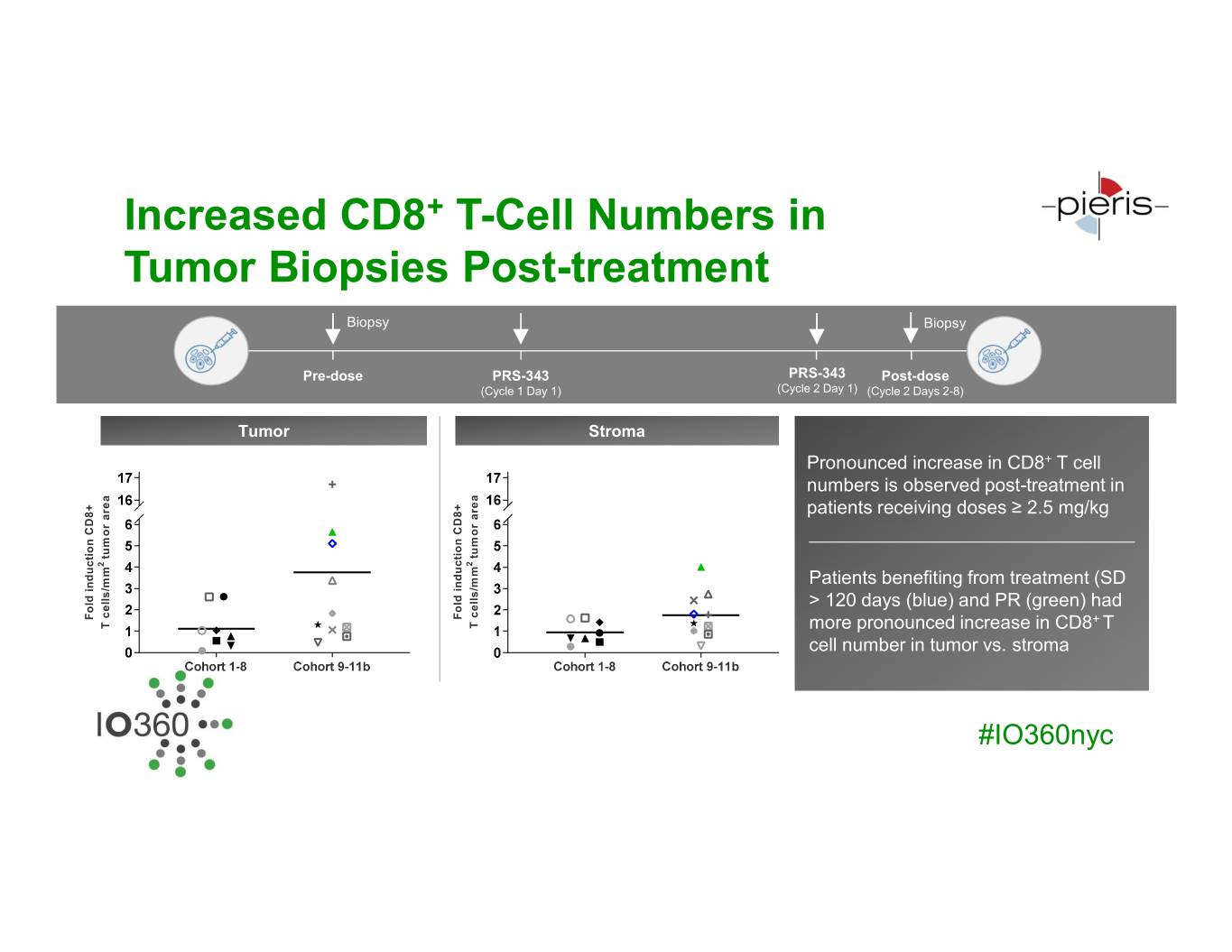
Increased CD8+ T-Cell Numbers in Tumor Biopsies Post-treatment Biopsy Biopsy Pre-dose PRS-343 PRS-343 Post-dose (Cycle 1 Day 1) (Cycle 2 Day 1) (Cycle 2 Days 2-8) Tumor Stroma Pronounced increase in CD8+ T cell numbers is observed post-treatment in patients receiving doses ≥ 2.5 mg/kg tumor area tumor tumor area tumor 2 2 Patients benefiting from treatment (SD > 120 days (blue) and PR (green) had Fold induction CD8+ induction Fold Fold induction CD8+ induction Fold + T cells/mm T T cells/mm T more pronounced increase in CD8 T cell number in tumor vs. stroma #IO360nyc
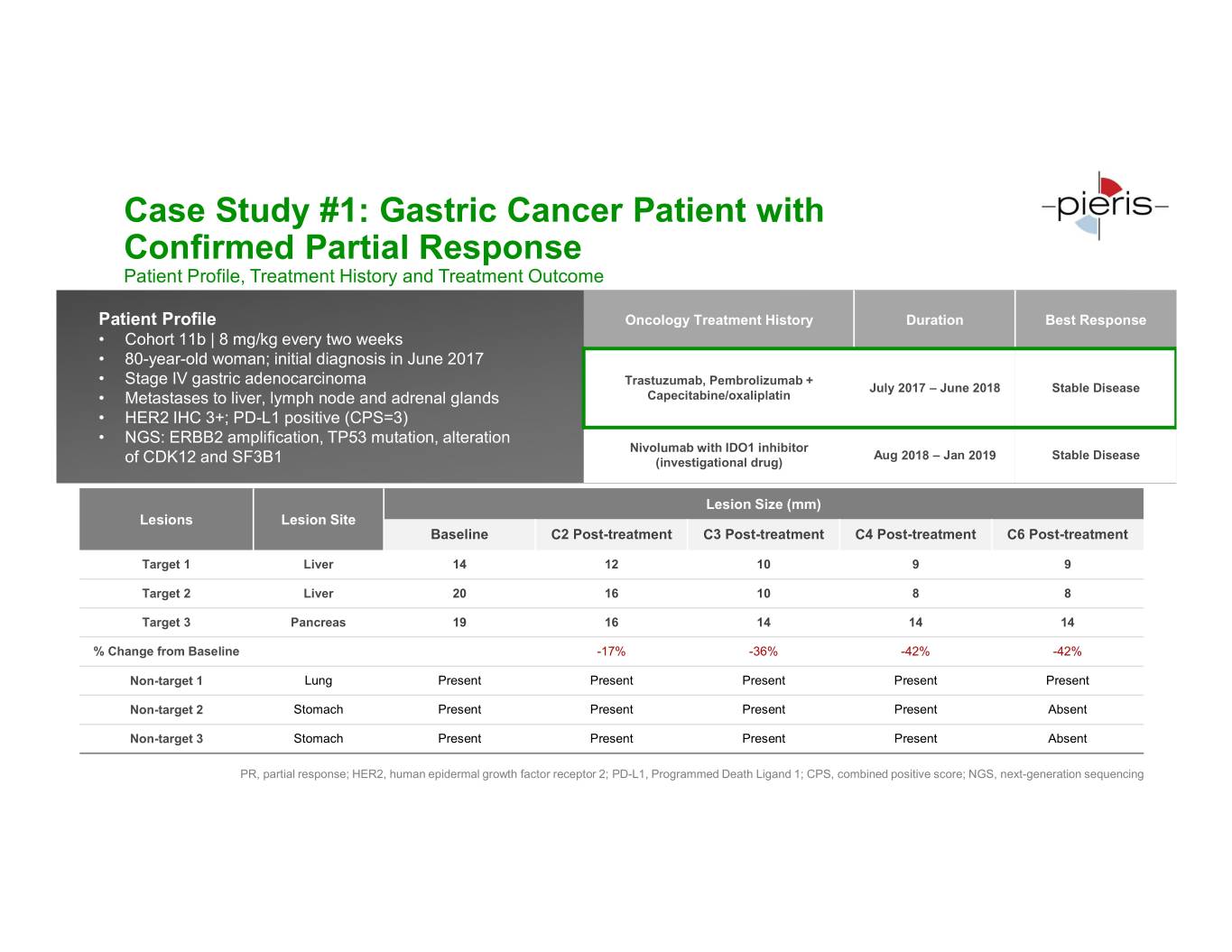
Case Study #1: Gastric Cancer Patient with Confirmed Partial Response Patient Profile, Treatment History and Treatment Outcome Patient Profile Oncology Treatment History Duration Best Response • Cohort 11b | 8 mg/kg every two weeks • 80-year-old woman; initial diagnosis in June 2017 • Stage IV gastric adenocarcinoma Trastuzumab, Pembrolizumab + July 2017 – June 2018 Stable Disease • Metastases to liver, lymph node and adrenal glands Capecitabine/oxaliplatin • HER2 IHC 3+; PD-L1 positive (CPS=3) • NGS: ERBB2 amplification, TP53 mutation, alteration Nivolumab with IDO1 inhibitor Aug 2018 – Jan 2019 Stable Disease of CDK12 and SF3B1 (investigational drug) Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C3 Post-treatment C4 Post-treatment C6 Post-treatment Target 1 Liver 14 12 10 9 9 Target 2 Liver 20 16 10 8 8 Target 3 Pancreas 19 16 14 14 14 % Change from Baseline -17% -36% -42% -42% Non-target 1 Lung Present Present Present Present Present Non-target 2 Stomach Present Present Present Present Absent Non-target 3 Stomach Present Present Present Present Absent PR, partial response; HER2, human epidermal growth factor receptor 2; PD-L1, Programmed Death Ligand 1; CPS, combined positive score; NGS, next-generation sequencing
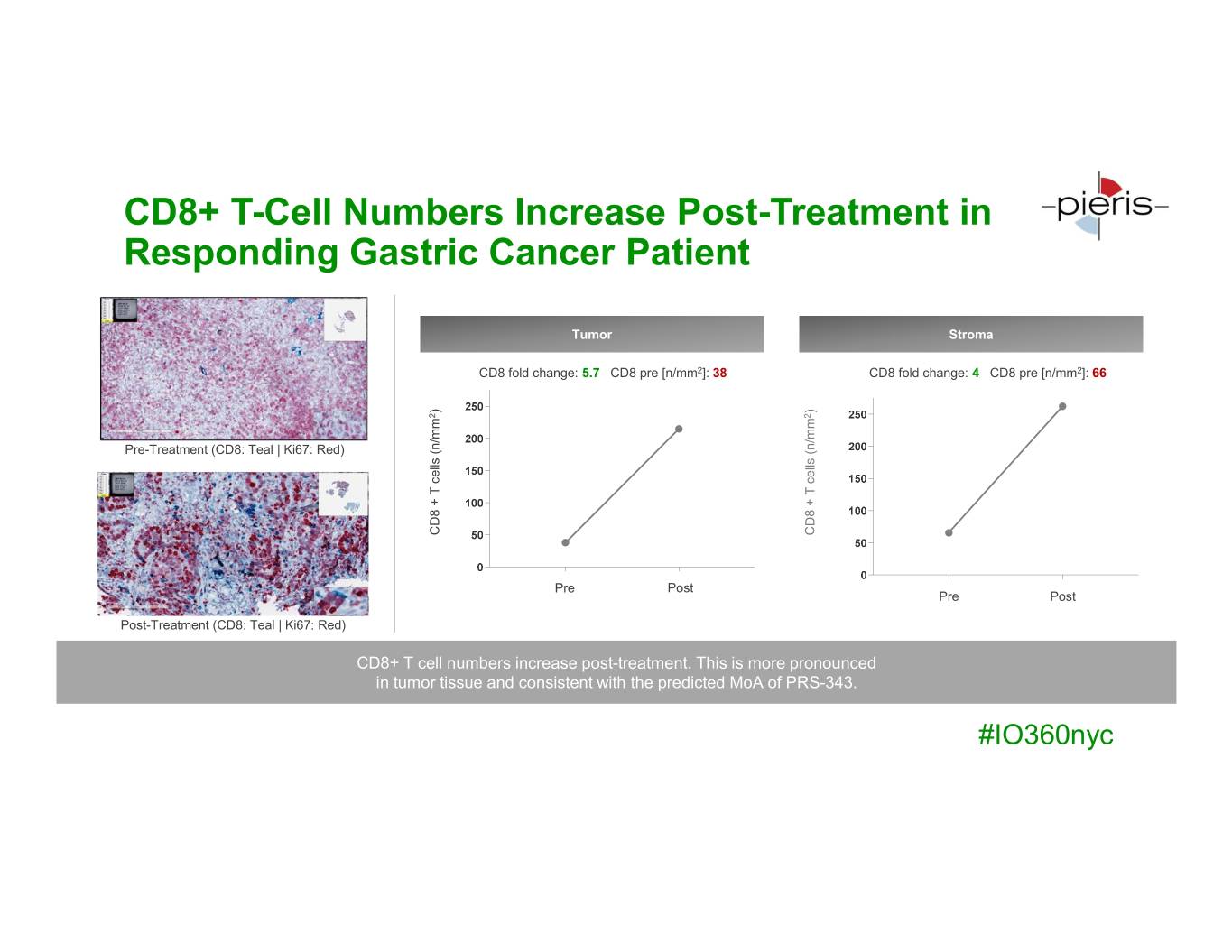
CD8+ T-Cell Numbers Increase Post-Treatment in Responding Gastric Cancer Patient Tumor Stroma CD8 fold change: 5.7 CD8 pre [n/mm2]: 38 CD8 fold change: 4 CD8 pre [n/mm2]: 66 ) ) 2 2 Pre-Treatment (CD8: Teal | Ki67: Red) CD8 + T T + CD8 (n/mmcells CD8 + T T +CD8 (n/mmcells Pre Post Pre Post Post-Treatment (CD8: Teal | Ki67: Red) CD8+ T cell numbers increase post-treatment. This is more pronounced in tumor tissue and consistent with the predicted MoA of PRS-343. #IO360nyc
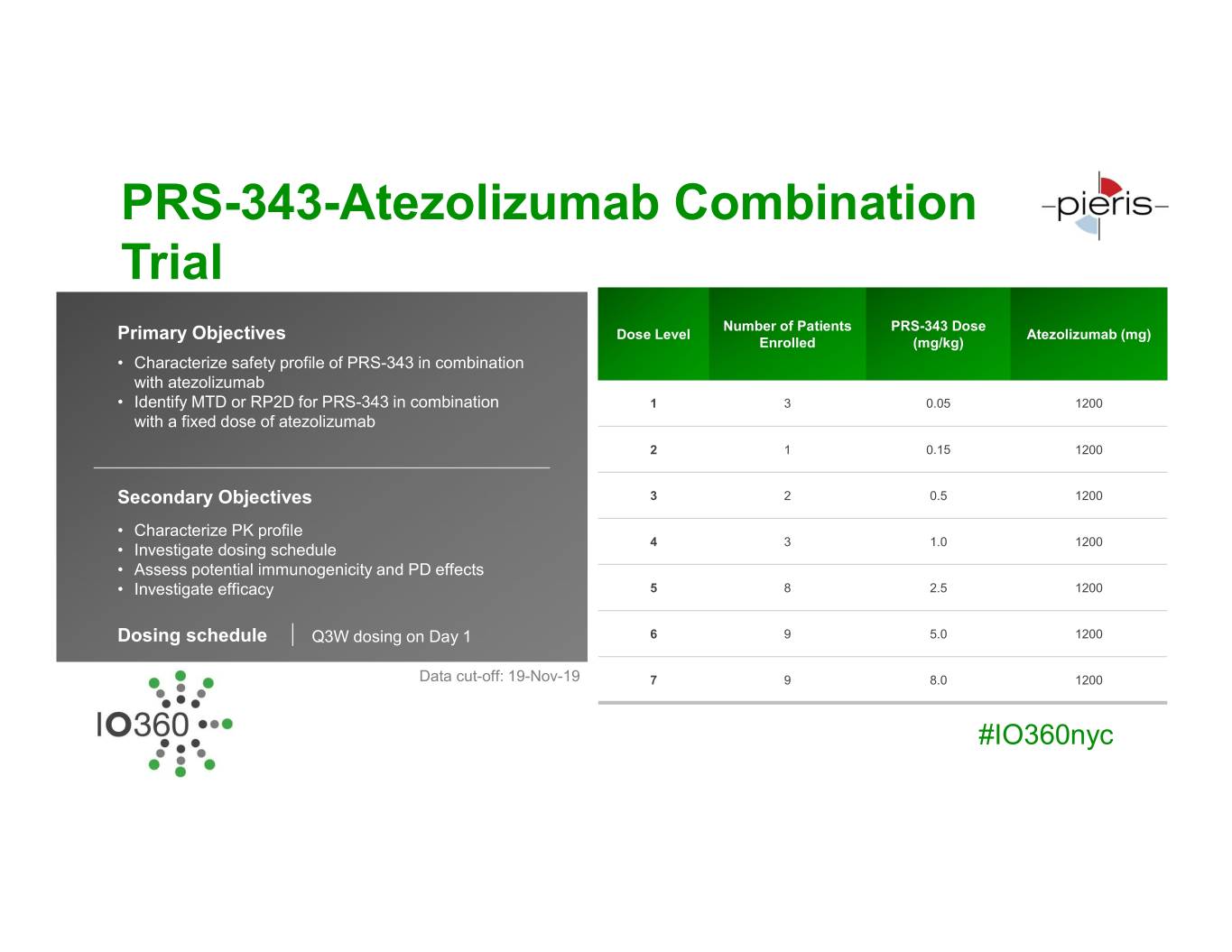
PRS-343-Atezolizumab Combination Trial Current Enrollment Number of Patients PRS-343 Dose Primary Objectives Dose Level Atezolizumab (mg) Enrolled (mg/kg) • Characterize safety profile of PRS-343 in combination with atezolizumab • Identify MTD or RP2D for PRS-343 in combination 1 3 0.05 1200 with a fixed dose of atezolizumab 2 1 0.15 1200 Secondary Objectives 3 2 0.5 1200 • Characterize PK profile • Investigate dosing schedule 4 3 1.0 1200 • Assess potential immunogenicity and PD effects • Investigate efficacy 5 8 2.5 1200 Dosing schedule Q3W dosing on Day 1 6 9 5.0 1200 Data cut-off: 19-Nov-19 7 9 8.0 1200 #IO360nyc
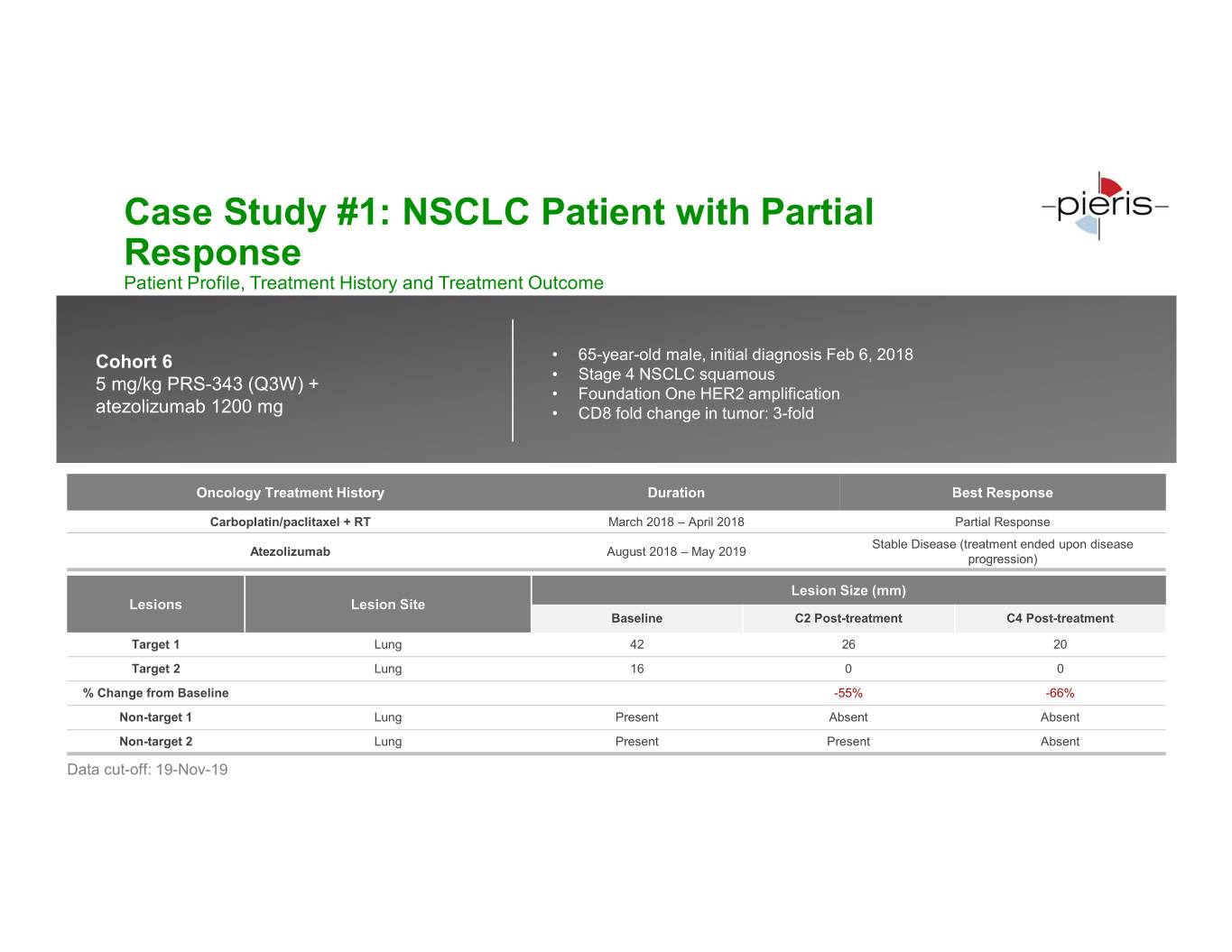
Case Study #1: NSCLC Patient with Partial Response Patient Profile, Treatment History and Treatment Outcome Cohort 6 • 65-year-old male, initial diagnosis Feb 6, 2018 • Stage 4 NSCLC squamous 5 mg/kg PRS-343 (Q3W) + • Foundation One HER2 amplification atezolizumab 1200 mg • CD8 fold change in tumor: 3-fold Oncology Treatment History Duration Best Response Carboplatin/paclitaxel + RT March 2018 – April 2018 Partial Response Stable Disease (treatment ended upon disease Atezolizumab August 2018 – May 2019 progression) Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C4 Post-treatment Target 1 Lung 42 26 20 Target 2 Lung 16 0 0 % Change from Baseline -55% -66% Non-target 1 Lung Present Absent Absent Non-target 2 Lung Present Present Absent Data cut-off: 19-Nov-19
Encouraging Anti-Tumor Activity with a HER2- Targeting 4-1BB-Based T-Cell Engager (PRS-343) in Solid Tumors Well-tolerated, with a good safety profile in all doses and schedules tested Demonstrated anti-tumor activity in heavily pre-treated patient population across multiple solid tumor types; treatment history indicative of 4-1BB-driven mechanism-of-action and synergy with PD-1/PD-L1 inhibition Showed a clear increase in CD8+ T cell numbers and proliferative index in the tumor microenvironment of responders Future studies are planned for continued development in defined HER2+ indications #IO360nyc